How Reverse Osmosis (RO) Works for Window Cleaning
Reverse osmosis, or RO, is one method that’s used to purify water when cleaning windows with a water fed pole (WFP). But how does it actually do that?
To understand reverse osmosis, lets take a quick look at osmosis, a natural process that occurs in nature. During osmosis, a less concentrated solution naturally migrates towards a more concentrated solution. A couple of examples of osmosis from nature are roots absorbing water from the soil or kidneys absorbing water from our blood.
Another example would be if you had two containers – one with salt water (i.e., a high concentration of salt) and one with fresh water (a low concentration of salt). If the two were separated by a semi-permeable membrane – i.e., a membrane that allows some molecules to pass but not others – the water with the lower salt concentration would move towards the water with the higher concentration.
Reverse osmosis, then, is the opposite. It’s when a more concentrated solution moves towards a less concentrated solution. However, while osmosis happens naturally, reverse osmosis requires applying energy to push the more concentrated solution through the semi permeable membrane.
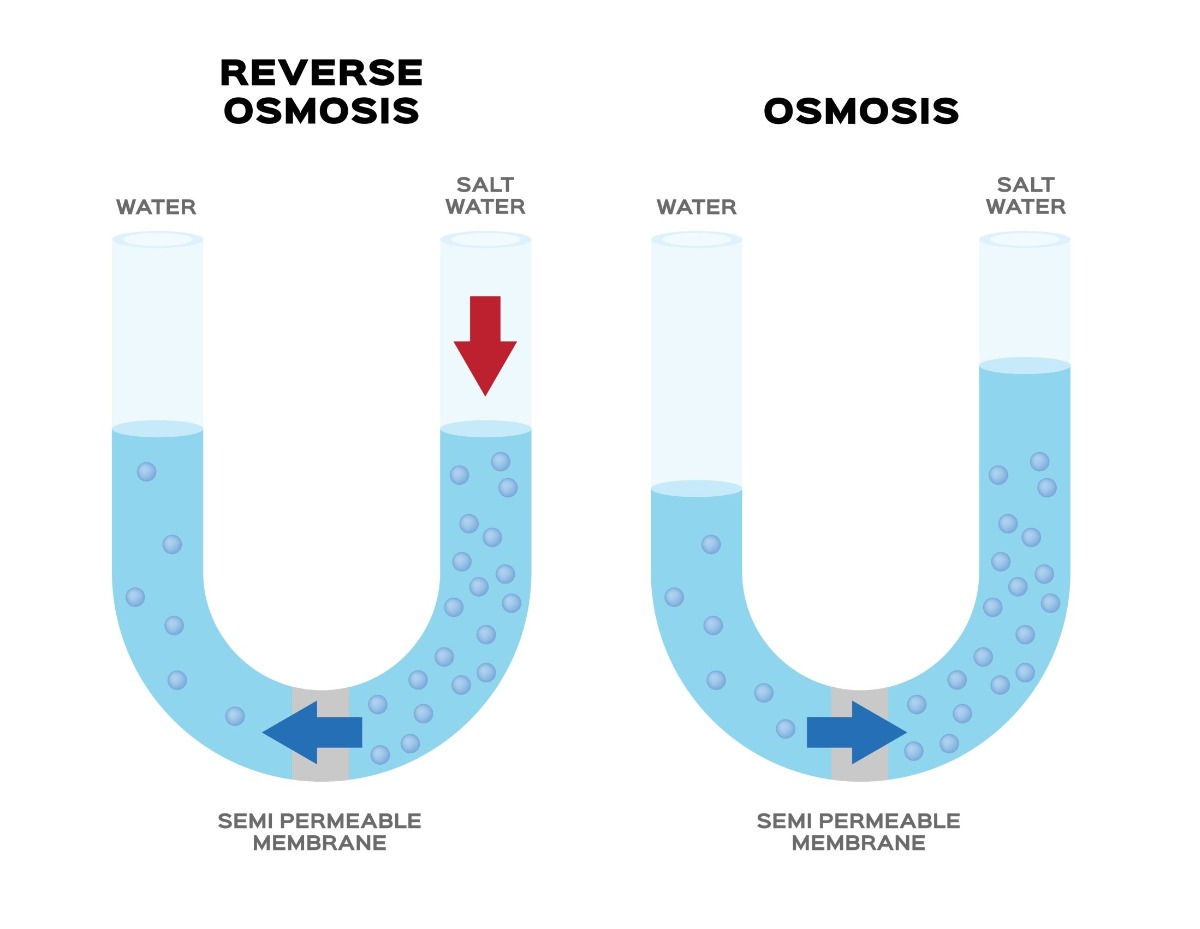
How a Reverse Osmosis Membrane Works
The RO membrane is semi-permeable. Without energy, water will just sit on top of the membrane. However, when put under pressure – and even just running tap water is enough – it will push the water through the droplets through the filter but not allow the calcium, magnesium, and other water spotting agents to pass through.
Why You Need a Three-Stage System
RO membranes for window cleaning systems are called Thin Film Composite Membranes (TFC or TFM) and can reject around 98% of standard contaminants. However, these RO membranes have an Achilles heel – chlorine. The chlorine that is in our water to keep us safe will also break down the membrane. That’s why RO systems require another filter, usually carbon, for the water to go through to remove the chlorine before it hits the reverse osmosis membrane.
This is why RO/DI is considered it “three-stage” system. First, the water goes through a carbon filter to remove the chlorine. Next, it hits the RO membrane, which removes 95% of the remaining contaminants, and then the DI resin canister removes the rest.

